Virus detection and analysis by nanopore and integration in a microfluidic device
1/2 doctoral allowance, 3 years, AAP 2019-1
Team: LAMBE UMR8587
Project leader: Laurent Bacri
Abstract:
In case of viral contamination, the targeted viruses must be detected very quickly, specifically, and with the lowest possible concentrations. Usual techniques do not detect a few thousand viral particles. They are long, expensive and sensitive to contaminations. To overcome these challenges, the virus must be detected at the single particle level in real time in the presence of the patient (point of care testing). We propose to use an electrical detection associated with nanopore technology and a microfluidic approach (B. Le Pioufle, IdA). Our strategy is to graft some antibodies to the surface of a solid state nanopore (G. Patriarche, C2N) to specifically capture viruses. In this thesis, we will study virus-like particles of rotavirus, viruses involved in acute gastroenteritis and responsible for hundreds of thousands of deaths per year (D. Poncet, I2BC).
The innovation of our project is to combine techniques already mastered to design an innovative sensor. Its design will require an interdisciplinary approach ranging from physical measurement and signal processing to surface chemistry, polymer science and the production of virus-like nanoparticles.
A first objective of this project is nanoscale surface chemistry to obtain homogeneous and specific grafting with controlled density. The main expected result is the capture of one or more virus-like particles at the nanopore vicinity, characterized by electrical or fluorescent measurements. This detection is based on a single particle technology and would increase the sensitivity to femto-mole. A second expected result is the design of a “lab-on-chip” integrating a filtration unit and a microfluidic partition. This approach could be generalized to the specific detection of biomarkers to design new diagnostic tools.
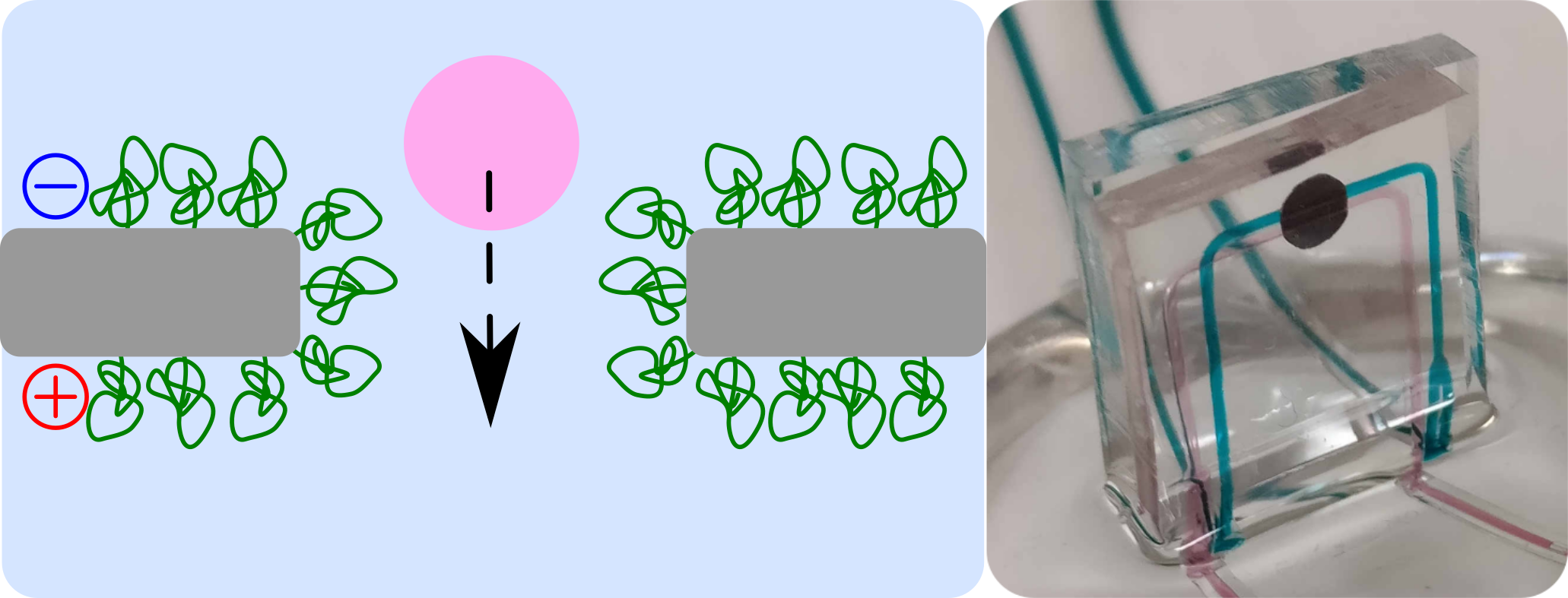
Nanoparticle detection and analysis by nanopore and integration in a microfluidic device.
(left) Transport of a thyroglobulin protein through functionnalized nanopore (J. Roman ACS Appl Mater Interfaces 2017, DOI: 10.1021/acsami.7b14717)
(right) Integration of a nanopore chips into a microfluidic device (J. Roman ACS Sensors 2018, DOI: 10.1021/acssensors.8b00700).
Themes:
Collaborations :
- Institut d’Alembert – Biomis Team
- C2N - Elaboration et Physique des Structures Epitaxiées